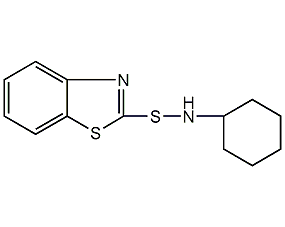
Structural formula
| Physical competition number | 0290 |
|---|---|
| Molecular formula | C13H16N2S2 |
| Molecular weight | 264.41 |
| label |
Accelerator CM CBS, Accelerator CM CBS, accelerator, Catalysts and auxiliaries |
Numbering system
CAS number:95-33-0
MDL number:MFCD00022872
EINECS number:202-411-2
RTECS number:DL6250000
BRN number:None
PubChem ID:None
Physical property data
1. Properties: white or light gray powder.
2. Relative density (g/mL, 20℃): 1.27-1.30
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (oC): 90-108
5. Boiling point (oC, normal pressure): Undetermined
6. Boiling point (oC, KPa): Undetermined Determined
7. Refractive index: Undetermined
8. Flash point (oC): Undetermined
9. Specific rotation (o): Undetermined
10. Autoignition point or ignition temperature (oC): Undetermined
11. Vapor pressure (mmHg, oC): Undetermined
12. Saturation Vapor pressure (kPa, oC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (oC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (% , V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: soluble in benzene; methylene chloride; tetracycline Carbon chloride; ethyl acetate; acetone, slightly soluble in ethanol and gasoline, insoluble in water.
Toxicological data
None
Ecological data
None
Molecular structure data
1. Molar refractive index: 77.63
2. Molar volume (cm3/mol): 208.9
3. Isotonic specific volume (90.2K ): 578.4
4. Surface tension (dyne/cm): 58.6
5. Polarizability (10-24cm3): 30.77
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 4.4
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: none
6. Topological molecule polar surface area 78.5
7. Number of heavy atoms: 17
8. Surface charge: 0
9. Complexity: 244
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14.NoThe number of stereocenters of certain chemical bonds: 0
15. The number of covalent bond units: 1
Properties and stability
1. Soluble in benzene, methylene chloride, carbon tetrachloride, ethyl acetate, acetone, slightly soluble in ethanol and gasoline, insoluble in water. It can gradually decompose when exposed to heat for a long time.
2. This product has low toxicity.
3. It can gradually decompose when exposed to heat for a long time.
Storage method
Store in a cool, dry and ventilated place, protected from moisture, fire and sun. Storage period is 6 months. It may clump easily during long-term storage, but it does not affect use.
Synthesis method
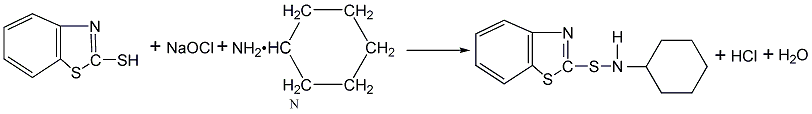
It is obtained by the reaction of accelerator M (2-thiol benzothiazole) and cyclohexylamine. Mix accelerator M and cyclohexylamine aqueous solution, add sodium hypochlorite dropwise under stirring to oxidize to obtain a crude product, separate the solid material, wash it with water until neutral, and dry it below 75°C to obtain the finished product.
Purpose
Mainly used in tires, rubber shoes, rubber hoses, tapes, cables, general industrial products, etc.
extended-reading:https://www.cyclohexylamine.net/butyltin-trichloridembtl-monobutyltinchloride/extended-reading:https://www.bdmaee.net/pc-cat-t120-catalyst-nitro/extended-reading:https://www.bdmaee.net/delay-catalyst-a-300/extended-reading:https://www.cyclohexylamine.net/trimerization-catalyst-pc-41-triazine-catalyst/extended-reading:https://www.cyclohexylamine.net/metal-delay-catalyst-strong-gel-catalyst/extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/24.jpgextended-reading:https://www.bdmaee.net/nt-cat-ea-104-catalyst-cas10027-41-9-newtopchem/extended-reading:https://www.cyclohexylamine.net/category/product/page/15/extended-reading:http://www.mimgu.com/archives/762extended-reading:https://www.cyclohexylamine.net/di-n-octyltin-oxide-dioctyltin-oxide-xie/
]]>